Chemists classify elements according to their position in the periodic table.
Periodicity is the term used to describe the repeating pattern of properties observed within the periodic table.
Image
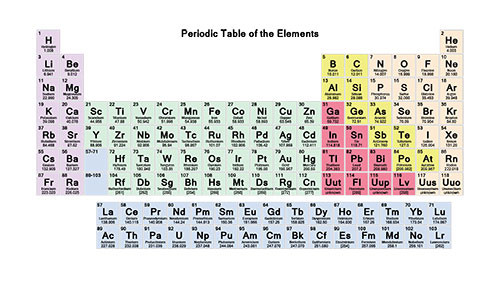
Image

Elements that have similar chemical properties are grouped in columns called groups (or families).
For example, alkali metals (the first column of elements) and noble gases (the last column of elements).
Metals are to the left of the heavy, stepped line in the 2nd picture, while nonmetals (except for hydrogen) are clustered to the right of the line.
Semimetals (or metalloids) are the elements with properties intermediate between those of metals and nonmetals and appear adjacent to the bold line.
- Acid Base Reactions
- Group VII, The Halogens
- Ligand Substitution Reaction
- Oxidation & Reduction
- Oxidation States
- Period 3 Elements
- Reaction Rates
- Reactions of Metal Aqua Ions
- Redox Reactions
- The Chelate Effect
- Transition Metals
- Trends in Group 1
- Trends in Group 2 Compounds
- Trends in Period Three of the Periodic Table
