Transition Metals
Transition metal characteristics arise from an incomplete d sub-level.
These characteristics include complex formation, high density, high melting points, formation of coloured ions, variable oxidation state and catalytic activity.
Definitions
- Complex: A metal ion surrounded by ligands.
- Ligand: an electron pair donor i.e. a molecule or ion joined onto the metal ion by a dative covalent bond to the metal.
- Coordination number: The number of atoms directly joined to a transition metal by a coordinate (dative covalent) bond.
Typical ligands
Unidentate H2O: :NH3 :Cl-
Bidentate C2O42- H2NCH2CH2NH2
Multidentate EDTA4-
Watch out for the chelate effect.
Shapes of transition metal complexes
Six-coordinate complexes are octahedral. e.g. Cu(H2O)62+ Co(NH3)63+
Four-coordinate complexes are usually tetrahedral e.g. CoCl42- but a few are square planar e.g. Ni(CN)42-.
Two-coordinate complexes are linear e.g. AgCl2-.
Why are transition metal complexes coloured?
Most transition metal colours are due to d-d electron transitions. The energy gap between the split d-orbitals corresponds to visible light (E=hf).
Some of the really strong colours are due to charge transfer (such as in MnO4-).
Changing the colour of transition metal complexes
Anything that changes the energy difference between the d-orbitals causes a change in colour:
- Oxidation state
- Ligand
- Coordination number
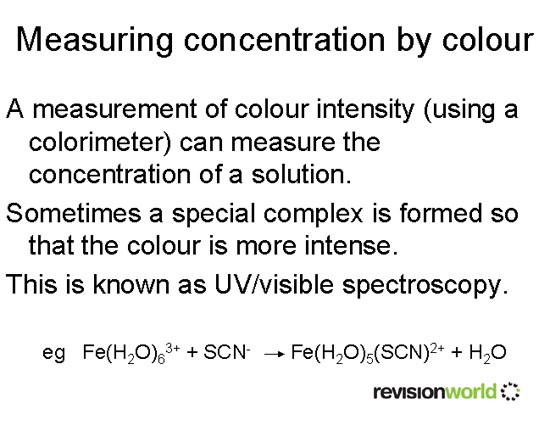
Reaction types
The reactions of the transition metals come under three headings (but sometimes more than one occurs at the same time!).
- Ligand substitution
One kind of ligand is replaced by another.
For example, Cu(H2O)62+ + 4Cl- → CuCl42- + 6H2O
- Hydrolysis (the acidity reaction)
One or more hydrogen ions is removed.
For example, Cu(H2O)62+(aq) + 2OH-(aq) → Cu(H2O)4(OH)2(s) + 2H2O(l)
- Redox
The oxidation state of the metal is changed by adding or removing one or more electrons
For example, Co(NH3)62+ → Co(NH3)63+ + e-
Variable Oxidation States
Transition elements are able to form more than one ion, each with a different oxidation state, by losing the 4s electrons and different numbers of 3d electrons.
When forming ions, the 4s electrons are lost first, before the 3d electrons.
Transition Metal Complexes
A ligand is a molecule or ion that bonds to a metal ion by donating one or more pairs of electrons.
The nucleophiles from organic chemistry and Lewis bases from more general inorganic chemistry fulfil the same role.
They a coordinate bond (a covalent bond in which both bonding electrons come from the same element) from the ligand to the transition metal ion.
A complex ion is a central metal ion surrounded by ligands.
The coordination number is the total number of coordinate bonds from ligands to the central transition metal ion.
A six-coordinate complex is octahedral.
A four-coordinate complex is usually tetrahedral (but can be square-planar).
A two-coordinate complex is linear (a good example is the range of silver complexes)
The effects of partially filled d-orbitals
In the partially filled d-orbitals of a transition metal complex there is an energy gap between the filled and unfilled orbitals.
Visible light is of the right energy to promote an electron from one energy level to the next.
The partially filled orbitals in transition metals allow them to change oxidation states readily and thus facilitate the progress of some reactions
Individual metals
Titanium
Mainly known for its chloride, TiCl4, as part of the Kroll process for extraction of titanium from its ores.
The chloride is covalently bonded. Titanium oxide, TiO2 , is used as a white pigment.
Vanadium

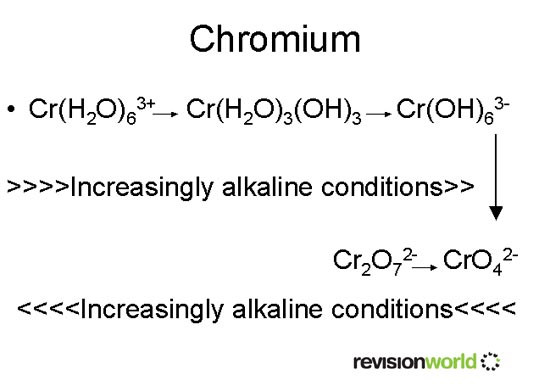
Chromium
Manganese

Iron
Fe(H2O)62+ is non-acidic in water (green).
Pure Fe(H2O)63+ is a lilac colour but on contact with water goes rusty brown.
Fe(H2O)62+ forms Fe(OH)2 (a green solid) with NaOH but it goes brown (forming Fe(OH)3 ) on standing in air.
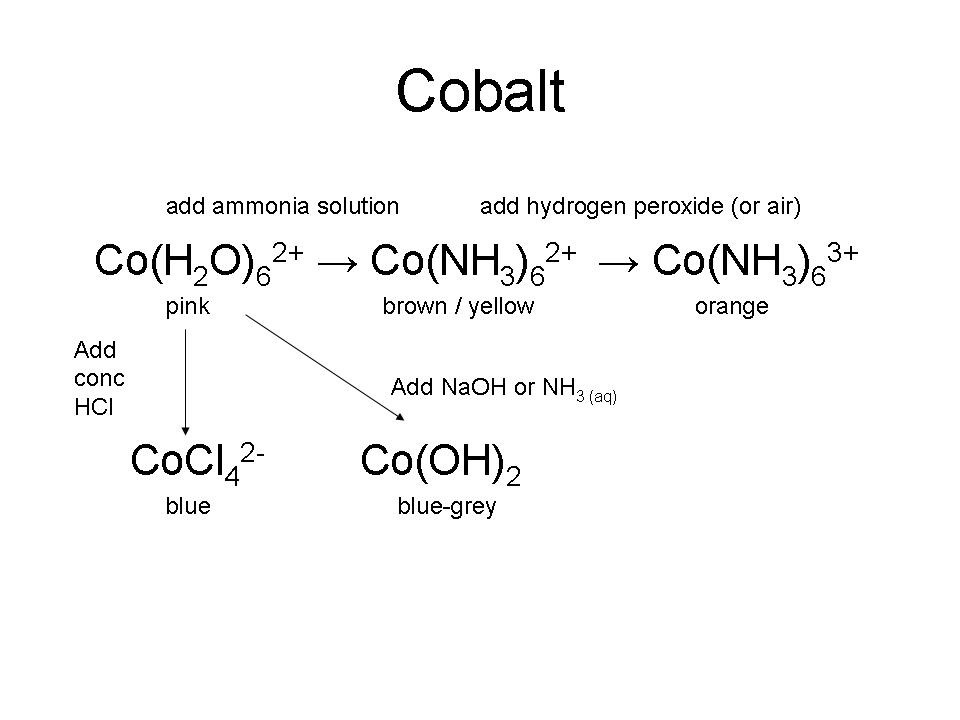
Cobalt
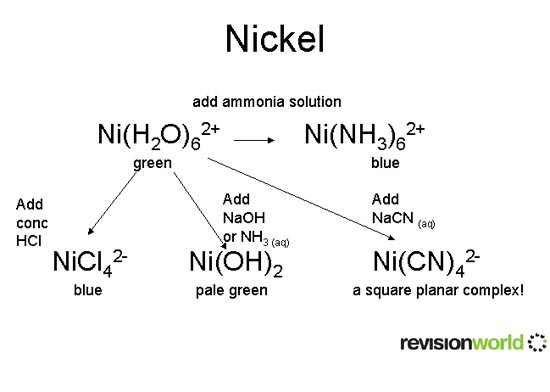
Nickel
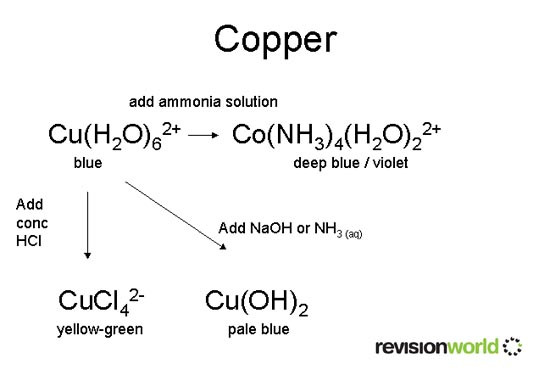
Copper
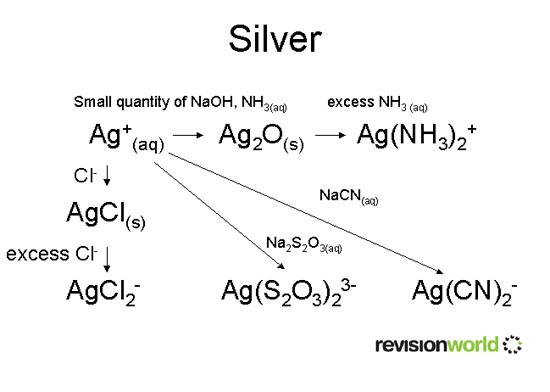
Silver
Uses of transition metals
V2O5 is used as a catalyst in the Contact Process (manufacture of sulfuric acid).
Cis-platin (a platinum complex) is a very effective anti-cancer drug.
Fe2+ is an important part of haemoglobin.
Ag(NH3)2+ is Tollen’s reagent (test for aldehydes).
Ag(S2O3)23- is formed during photographic processing.
Ag(CN)2- is used in electroplating.
