Trends in Period Three of the Periodic Table
Image
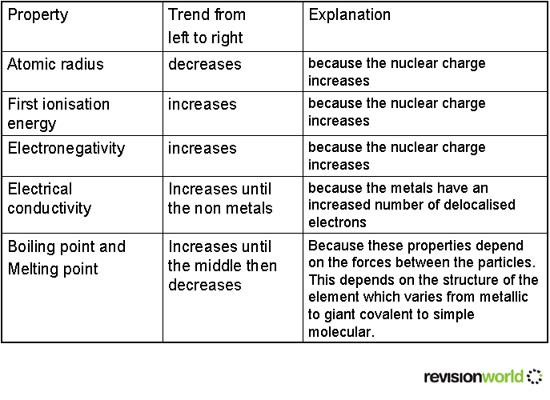
Groups I, II and III are metals and so metallic bonding is used.
Group IV is a macromolecule and so has a very large melting point.
Groups V, VI, VII are molecules and so we rely on temporary dipole-dipole attractions.
Phosphorus is P4 and so has a reasonably large temporary dipole.
Sulphur is S8 and so has a very large temporary dipole.
Chlorine is Cl2 and so has a fairly small temporary dipole.
Going to argon, we notice that it is formed of single atoms and so it has a very small temporary dipole.
