Ionic and Covalent Structures
This section looks at ionic and covalent compounds.
Structure of ionic compounds
Giant structures are formed by compounds containing ionic bonds. These structures are held together by strong electrostatic forces of attraction between the oppositely charged ions. These forces act in all directions throughout the lattice.
The diagram below represents a typical giant ionic structure, sodium chloride.
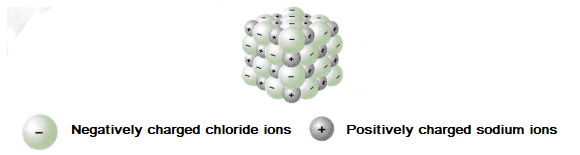
The empirical formula of the compound can be worked out by the ratio of each ion present in the structure. In the diagram above there are equal numbers of sodium ions and chloride ions. This means the empirical formula is NaCl.
Structure of covalent compounds
A covalently bonded substance may consist of:
- Small molecules and simple molecular structures (e.g. Cl2, H2O and CH4)
- Large molecules (e.g. polymers)
- Giant covalent structures (e.g. diamond, graphite and silicon dioxide).
Small molecular structures
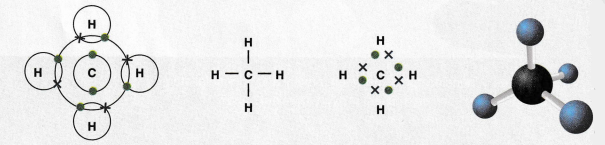
We can represent the bonding between hydrogen and carbon in methane in several ways below.
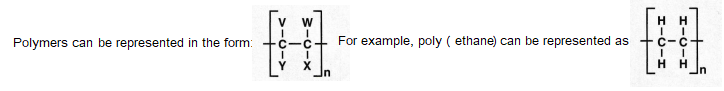
Large molecules
V,W,X and Y represent the atoms bonded to the carbon atoms.
Giant covalent structures
The diagram below is a giant covalent structure (silicon dioxide).
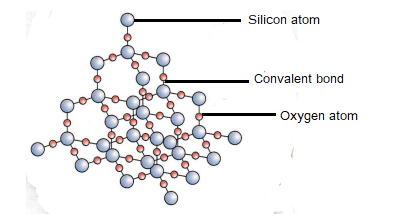
The formula of silicon dioxide is SiO2 – this can be deduced by looking at the ratio of Si to O atoms in the diagram above.
A chemical bond is not a solid object as depicted in some models. It is actually an attraction between particles. The relative size of different atoms is often not shown when drawing diagrams.
Glossary of terms
Empirical formula – The simplest whole number ratio of each kind of atom present in a compound.
Polymer – A large, long chained molecule.
The video below explains ionic and covalent bonding.
You can find more information about Ionic bonding here
