Properties of Small Molecules and Polymers
This section looks at the properties of compounds, including small molecules, polymers and giant covalent structures.
Properties of Small Molecules
Substances made up of small molecules are usually gases or liquids at room temperature. They have relatively low melting and boiling points because there are weak (intermolecular) forces that act between the molecules. It is these weak forces and not the strong covalent bonds that are broken when the substance melts or boils.
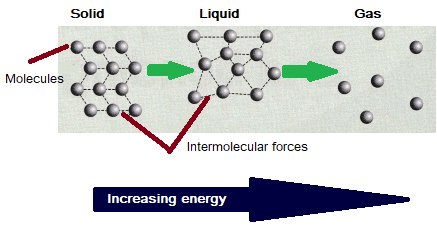
Substances made up of small molecules do not normally conduct electricity. This is because the molecules do not have an overall electric charge or delocalised electrons.
Polymers
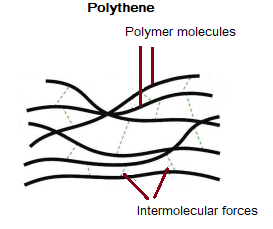
Polymers are large molecules made up of atoms joined together by strong covalent bonds. The intermolecular forces between polymer molecules are much stronger than in small molecules because the molecules are larger. This is why most polymers are solid at room temperature.
Giant covalent structures
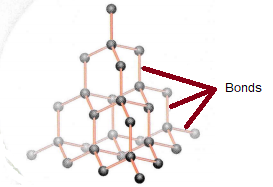
Substances with a giant covalent structure are solids at room temperature. They have relatively high melting and boiling points. This is because there are lots of strong covalent bonds that need to be broken.
The video below looks at the properties of simple molecular substances and giant covalent structures.
You can find out more about states of matter here
