States of Matter
This section looks at states of matter and how they can be interconverted.
Solids, liquids and gases
The three main states of matter are solids, liquids and gases. Individual atoms do not have the same properties as these bulk substances. The diagram below shows how the particles in the different states of matter are arranged and how they can be interconverted.
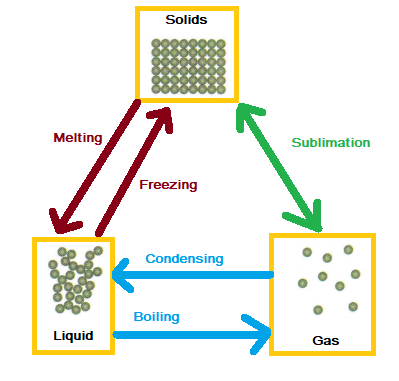
The changes are physical because particles are either gaining or losing energy and are not undergoing a chemical reaction. Particles in a gas have more energy than in a liquid and particles in a liquid have more energy than in a solid.
Melting and freezing occur at the same temperature. Condensing and boiling also occur at the same temperature. The amount of energy needed to change state depends on the strength of the forces between the particles of the substance. The stronger the forces between the particles, the higher the melting and boiling points of the substance.
Properties of ionic compounds
High melting and boiling points – There are lots of strong bonds throughout an ionic lattice which require lots of energy to break.
Electrical conductivity – Ionic compounds conduct electricity when molten or dissolved in water because the ions are free to move and carry the charge. Ionic solids do not conduct electricity because the ions are in a fixed position and are unable to move.
Glossary of terms
Intermolecular forces – The weak forces of attraction that occur between molecules.
The video below explains states of matter.
You can find out more about the properties of small molecules and polymers here
