Structure and Bonding of Carbon
This section looks at the structure and bonding of carbon, including diamonds, graphite, graphene and fullerenes.
Carbon has four different structures.
Diamond
Diamond is a giant covalent structure where each carbon atom is bonded to four others.

In diamond, there are lots of very strong covalent bonds so diamonds are hard and have a very high melting point. It’s these properties that make diamonds ideal for making cutting tools. Diamonds also contain no electrons so they don’t conduct electricity.
Graphite
Like diamond, graphite is also a giant covalent structure, with each carbon atom forming three covalent bonds, resulting in layers of hexagonal rings in carbon atoms. Carbon has four electrons in its outer shell and as only three are used for bonding the other one is delocalised.
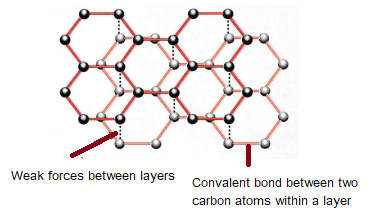
The layers in graphite are able to slide over each other because there are only weak intermolecular forces holding them together. This is why graphite is soft and slippery. These properties make graphite suitable for use as a lubricant.
Like diamond there are lots of strong covalent bonds in graphite so it has a high melting point.
The delocalised electrons allow graphite to conduct electricity and heat.
Graphene and fullerenes
Graphene is a single layer of graphite and so it is one atom thick.

Fullerenes are molecules made up of carbon atoms and they have hollow shapes. The structure of fullerenes is based on hexagonal rings of carbon atoms but they may also contain rings with five or seven carbon atoms.
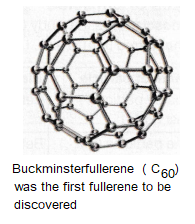
Carbon nanotubes are cylindrical fullerenes. Fullerenes have a high tensile strength, they are electrical and thermal conductivity.
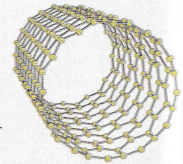
Fullerenes can be used for drug delivery into the body as lubricants and for reinforcing materials e.g. tennis rackets.
The video below explains the structure of diamond and graphite.
You can find out more about the structure and bonding of metals and alloys here
