Structure and Properties of Metals and Alloys
This section looks at the structure and properties of metals and alloys.
Structure and properties of metals
Metals have giant structures. Metallic bonding is strong meaning that most metals have very high melting and boiling points.
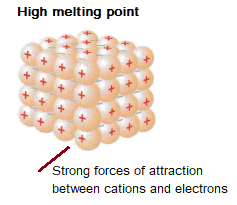
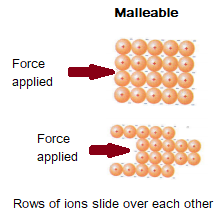
The layers are able to slide over each other, which means that metals can be bent and shaped.
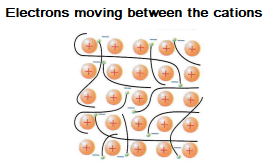
Metals are good conductors of electricity because the delocalised electrons are able to move.
The delocalised electrons also transfer energy meaning that they are good thermal conductors.
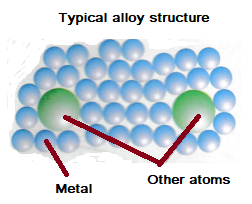
Alloys
Most metals we use are alloys. Many pure metals such as aluminium, gold and iron are too soft for many uses and so are mixed with other materials (usually other metals) to make alloys.
The different sizes of atoms in alloys make it difficult for the layers to slide over each other. This is why alloys are stronger than pure metals.
Glossary of terms
Alloys – A mixture of two or more metals, or a mixture of a metal and a non-metal.
Fullerene – A molecule made or carbon atoms arranged as a hollow sphere.
Nanotube – A molecule made of carbon atoms arranged in a tubular structure,
High tensile strength – Does not break easily when stretched.
The video below explains the properties of alloys.
You can find out more about the structure and bonding in carbon here
